Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tochiyama, Osamu*
JNC TJ8400 2000-044, 53 Pages, 2000/02
To estimate the polyelectrolyte effect and the effect of the heterogeneous composition of humic acids, the complex formation constants of Eu(III) and Ca(II) with Aldrich humic acid and polyacrylic acid were obtained, for Eu(10 to 10
to 10 M) by solvent extraction with TTA and TBP in xylene, for Ca (10
M) by solvent extraction with TTA and TBP in xylene, for Ca (10 M) with TTA and TOPO in cyclohexane and for Ca(10
M) with TTA and TOPO in cyclohexane and for Ca(10 M) by using ion-selective electrode. By defining the apparent formation as
M) by using ion-selective electrode. By defining the apparent formation as  = [MR
= [MR ]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR
]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR ] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log
] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO
have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO and NaCl. Log
and NaCl. Log of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log
of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log
increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log , the decrease in log
, the decrease in log of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log
of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log of polyacrylate, log
of polyacrylate, log of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log
of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log  of humate by 0 to 0.8. The dependence of log
of humate by 0 to 0.8. The dependence of log of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
*; Sato, Haruo; *
JNC TN8400 99-059, 59 Pages, 1999/10
Organic acids in groundwater are considered to form complexes and increase the solubility of radionuclides released from vitrified waste in a high-level radioactive waste (HLW) repository. To ivestigate whether the solubility of samarium (Sm) is influenced by organic substances, we measured Sm solubility in the presence of different organic substances and compared those values with results from thermodynamic predictions. Humic acid (Aldrich) is commercially available and soluble organic matter originated from bentonite were used as organic substances in this study. Consequently, the solubility of Sm showed a tendency to apparently increase with icreasing the concentration of humic acid, but in the presence of carbonate, thermodynamic predictions suggested that the dominant species are carbonate complexes and that the effect of organic substances are less than that of carbonate. Based on total organic carbon (TOC), the increase of Sm solubility measured with humic acid (Aldrich) was more significant than that in the case with soluble organic matter originated from bentonite. Since bentonite is presumed to include also simple organic matters of which stability constant for forming complexes is low, the effect of soluble organic matter originated from bentonite on the solubility of Sm is eonsidered to be less effective than that of humic acid (Aldrieh). Experimental values were compared with model prediction, propsed by Kim, based on data measured in a low pH region. Tentatively we calculated the increase in Sm solubility assuming complexation with humic acid. Trial calculations were carried out on the premise that the complexation reaction of metal ion with humic acid is based on neutralization process by 1-1 complexation. In this process, it was assumed that one metal ion coordinates with one unit of complexation sites which number of proton exchange sites is equal to ionic charge. Consequently, Kim's model indicated that carbonate complexes should be dominant ...

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO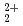 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Lothenbach, B.*; Ochs, M.*; Wanner, H.*; Yui, Mikazu
JNC TN8400 99-011, 340 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of palladium Pd, lead Pb, tin Sn, antimony Sb, niobium Nb and bismuth Bi in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system of high-level radioactive wastes. Besides treating hydrolysis in detail, this report focuses on the formation of complexes or compounds with chloride, fluoride, carbonate, nitrate, sulfate and phosphate. Other important inorganic ligands (sulfide for lead and antimony, ammonia for palladium) are also included. In this study, the specific ion interaction theory (SIT) approach is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.
Nakayama, Shinichi; Yamaguchi, Tetsuji; Nagano, Tetsushi
JAERI-Conf 97-002, p.13 - 14, 1997/02
no abstracts in English
; Aose, Shinichi; ;
PNC TN8410 95-313, 28 Pages, 1995/10
THe coordination properties of the lanthanide (La, Ce, Pr, Nd, Sm and Eu) complexes in lanthanide/TBP (tributylphosphate), lanthanide/CMPO (octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide) and lanthanide/CMPO/TBP systems were investigated by the NMR (nuclear magnetic resonance) measurements. The numbers of the coordinated CMPO and TBP to the lanthanide ion were estimated about three and two in the lanthanide/CMPO and lanthanide/TBP systems, respectively. It is considered that TBP and CMPO coordinate to the lanthanide (III) ion in the monodentate and the bidentate manners, respectively. In the lanthanide/CMPO/TBP system, 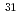 P-NMR spectra suggested that CMPO coordinates to lanthanide(III) ion directly in the bidentate mode, but TBP doesn't exist within the first coordination sphere and coordinates to the lanthanide(III) ion from beyond the first coordination sphere. The activation parameters for the ligand exchange reactions calculated by the CBS (complete bandshape) method suggest that the ligand exchange reactions in the lanthanide (Pr and Eu)/TBP and lanthanide (La, Pr and Sm)/CMPO systems proceed through either the associative (A) mechanism or the dissociative (D) mechanism with an ordering into the second coordination sphere. In the lanthanide (Pr and Sm)/CMPO/TBP systems, it was shown that the CMPO exchange reactions proceed through the mechanism with an ordering into the second coordination sphere, which is caused by TBP in the systems.
P-NMR spectra suggested that CMPO coordinates to lanthanide(III) ion directly in the bidentate mode, but TBP doesn't exist within the first coordination sphere and coordinates to the lanthanide(III) ion from beyond the first coordination sphere. The activation parameters for the ligand exchange reactions calculated by the CBS (complete bandshape) method suggest that the ligand exchange reactions in the lanthanide (Pr and Eu)/TBP and lanthanide (La, Pr and Sm)/CMPO systems proceed through either the associative (A) mechanism or the dissociative (D) mechanism with an ordering into the second coordination sphere. In the lanthanide (Pr and Sm)/CMPO/TBP systems, it was shown that the CMPO exchange reactions proceed through the mechanism with an ordering into the second coordination sphere, which is caused by TBP in the systems.
;
PNC TN8420 95-002, 140 Pages, 1995/01
In the field of uranium ore refining and spent fuel reprocessing, a lot of extractants capable of recovering actinides have been innovated, and some of which have been developed in actual industrial scale processes. Extractability against trivalent actinides on each extractants were evaluated in this report. And as for the compounds which showed excellent extractability for Americium, their extraction characteristics for trivalent actinides, such as extraction behavior, mechanism, ligand structure and conformation of complexes, were evaluated, and filed as data-base. Based on these works, some of extractant were newly identified as candidate to apply Americium recovering. The most appropriate compounds for trivalent actinide separation can be generally found in the neutral bidentate organic compound groups: Especially, some derivatives belongs to Diphosphineoxide, Carbamoyl-methylphosphine oxide and Propandiamide were superior to give sufficiently excellent property even in higher nitric acid(1 3M) solutions. Innovative research towards developping new extractants and extraction system seems to be directed to ; (1)investigate new types of extractant sustained by a different kind of complexing mechanism, (2)evaluate synergistic effect of adducts, diluents even including conventional extractants.
3M) solutions. Innovative research towards developping new extractants and extraction system seems to be directed to ; (1)investigate new types of extractant sustained by a different kind of complexing mechanism, (2)evaluate synergistic effect of adducts, diluents even including conventional extractants.